
Established in 2004, CIDP (Centre International de Développement Pharmaceutique) is an international Contract Research Organisation (CRO) that carries out high R&D activities for pharmaceutical, medical device, nutraceutical and cosmetic industries.
CIDP ROMANIA
Founded in 2010, CIDP Romania is located at the heart of Bucharest, the capital and largest city of Romania, close to both Metro and Bus stations and 25 minutes from Henri Coanda International Airport and occupies an office space of 875 m².

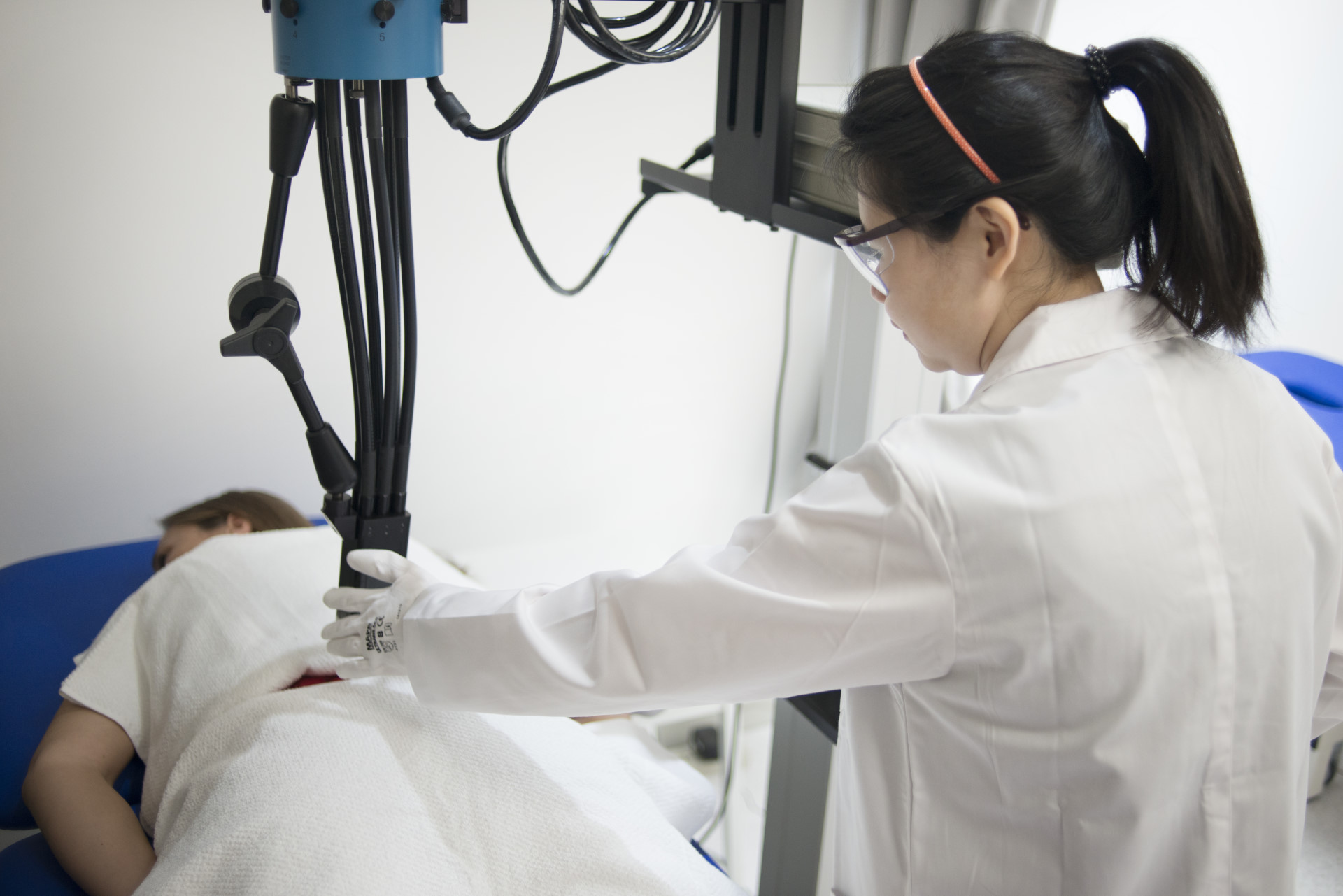
CIDP Romania boasts a robust team consisting of 35 dedicated team members, primarily comprising:
- Principal and Co-Investigators
- Clinical Trial Scientists
- Volunteers Recruitment Team
- Regulatory Advisors
- QHSE Specialists
In addition, CIDP has a pool of investigators specialized in dermatology, pediatrics, ophthalmology, gynecology, etc.
Volunteers
Bucharest is the fourth-largest city in the European Union, with a population estimated at 1.8 million.
- The main skin photo types found are I, II, III, & IV
- The database of volunteers comprises around 9,000 active volunteers.
In terms of Pathologies, a wide variety of skin disorders can be recruited, among which the most common ones are acne, dermatitis, rosacea, lentigo, melasma, age spots, dandruff and acrochordon known as papilloma.

Investigational Products
Investigational Products (IP) shipped from outside the EU to Romania are generally subject to VAT of 19% when arriving at the Customs in Romania. However, this process may be avoided if the invoice accompanying the products mentions that they are not for Commercial Use ( Clinical Trial Use only) and the declared value including Transport Cost is less than €10.
Allow 5 working days for shipment time when using an express courier company.

Regulatory
There is no legal requirement for obtaining an approval from the regulatory authorities (Romanian law) or IEC prior the start of a study for cosmetic and exploratory studies in Romania.
There is also the possibility of approaching the IEC of CIDP for an approval and the timeframe is 30 days.

Our Gallery
Reception

Team

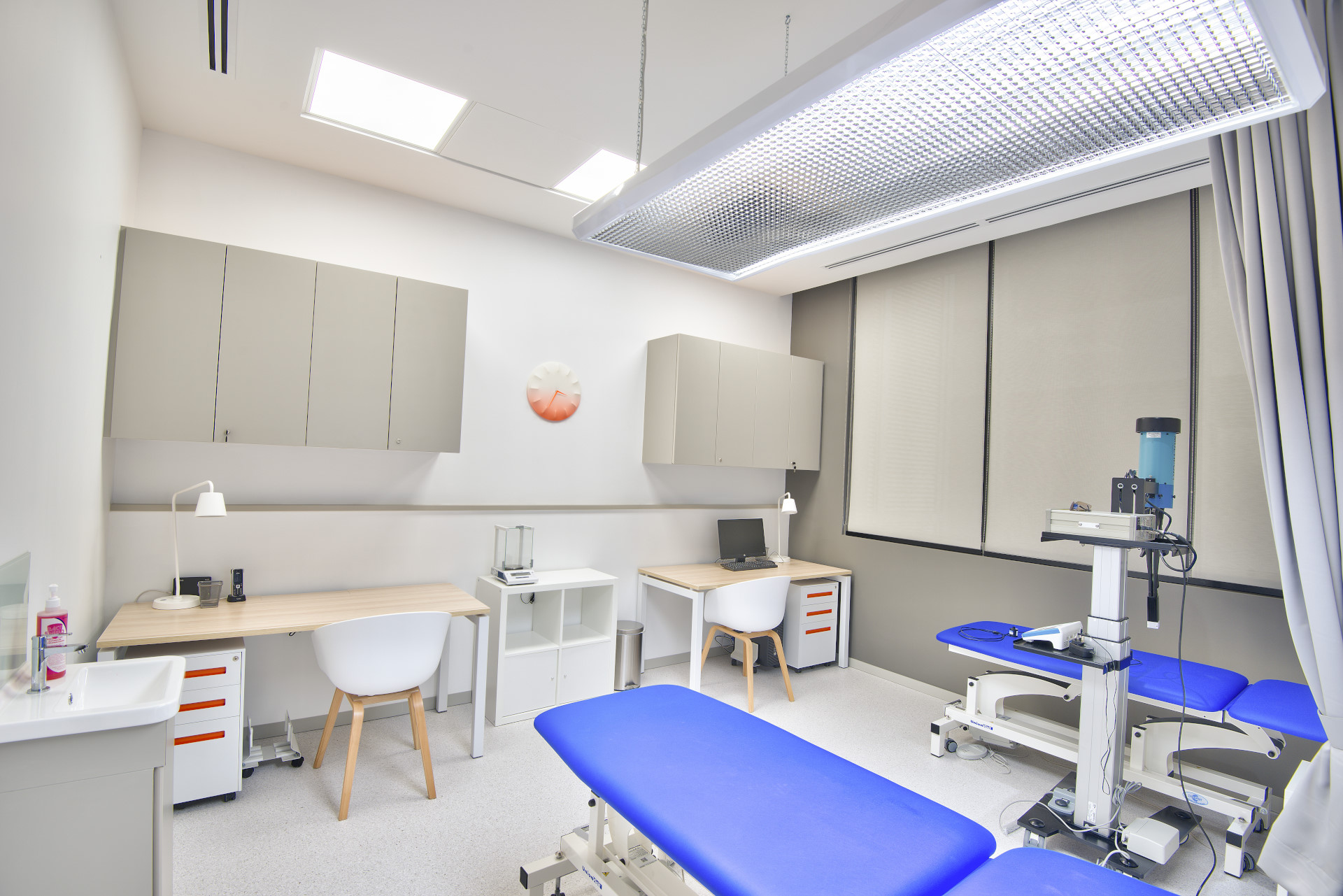
Evaluation Room
Waiting Room

Equipment Bank

Bucharest office







