Established in 2004, CIDP (Centre International de Développement Pharmaceutique) is an international Contract Research Organisation (CRO) that carries out high R&D activities for pharmaceutical, medical device, nutraceutical and cosmetic industries.
CIDP INDIA
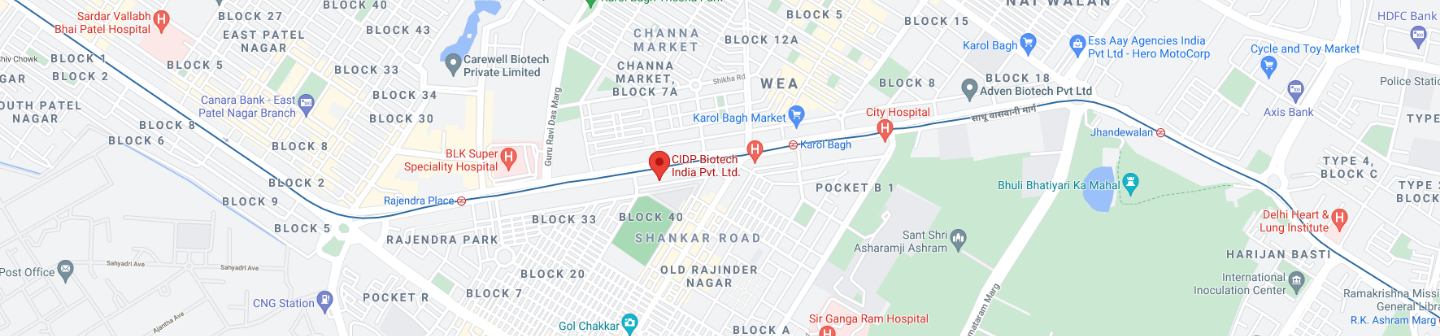
Founded in 2011, CIDP India is located in the heart of the city of New Delhi, at Rajender Nagar, which is in close proximity to the multiple metro stations, bus stands, railway stations and about 20 minutes away from Indira Gandhi International Airport. With an office space spanning to 6300 square feet, comprising of three main blocks with clinical and administrative areas.

CIDP India comprises of a team of 35 staff which comprises mainly:
- Principal Investigator and Co-Investigators
- Clinical Trial Managers, Monitors and Coordinators
- Quality Health Safety and Environment (QHSE) team
- Subject Recruitment Manager
- Regulatory advisors
In addition, CIDP collaborates with many external Investigators, Ophthalmologists and Pediatrician for the conduct of studies.
Volunteers
With a population of 31 million in New Delhi, our in-house database contains around 10000 volunteers of phototypes III- VI .
Delhi enjoys 5 seasons (Summer, Monsoon, Autumn, Winter and Spring) and studies can be planned throughout the year without major disturbances.
Common Skin Pathologies: Acne, Melasma, Dark spots (PIHP, freckles, solar lentigines), Vitiligo, Scalp psoriasis, Eczema, Seborrhoea and pollution induced skin damage.

Investigational Products
CIDP strongly recommends sending the investigational product through your company if you have a branch in India as this can ease the process of investigational product reception into India. The product shipment is normally initiated after obtaining the Ethics Clearance approval.
Investigational products shipment requires Import License (Pharma products) from DCGI (Drugs Controller General of India) and a no-objection certificate from ADC (for cosmetic products).
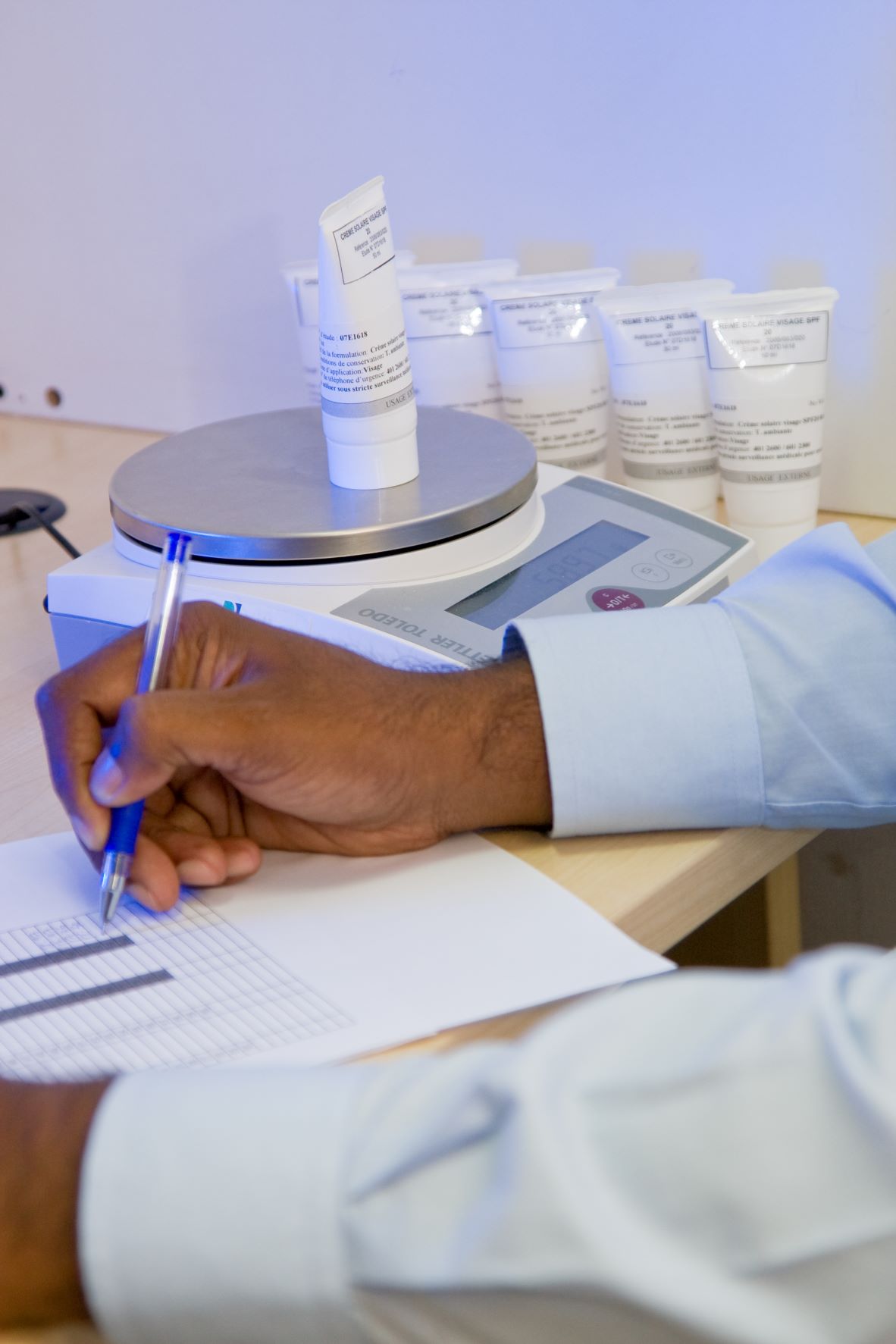
Regulatory
Every clinical study conducted at CIDP India is in accordance with ethical standards, ICMR– Ethical guidelines for biomedical research on human participants, ICH-GCP and local legislations( The New Drugs & Clinical Trials Rules, 2019 ). It may take 15 days-30 days for local EC approval and 90 days for DCGI approval.

PARTNERSHIP
- Maulana Azad Medical College
- All India Institute of Medical College (AIIMS)
- Lady Hardinge Medical College
- Sir Ganga Ram Hospital
- Safdarjung Hospital
- Action Cancer Hospital
- BLK Hospital
Our Gallery
Reception

Waiting Room

Investigator Room
Sample Room

Shampoo Room

India
32-B, First floor, Rajendra Nagar, Pusa Road, New Delhi -110005